The 12 Hallmarks of Aging: How Do They Affect Aging
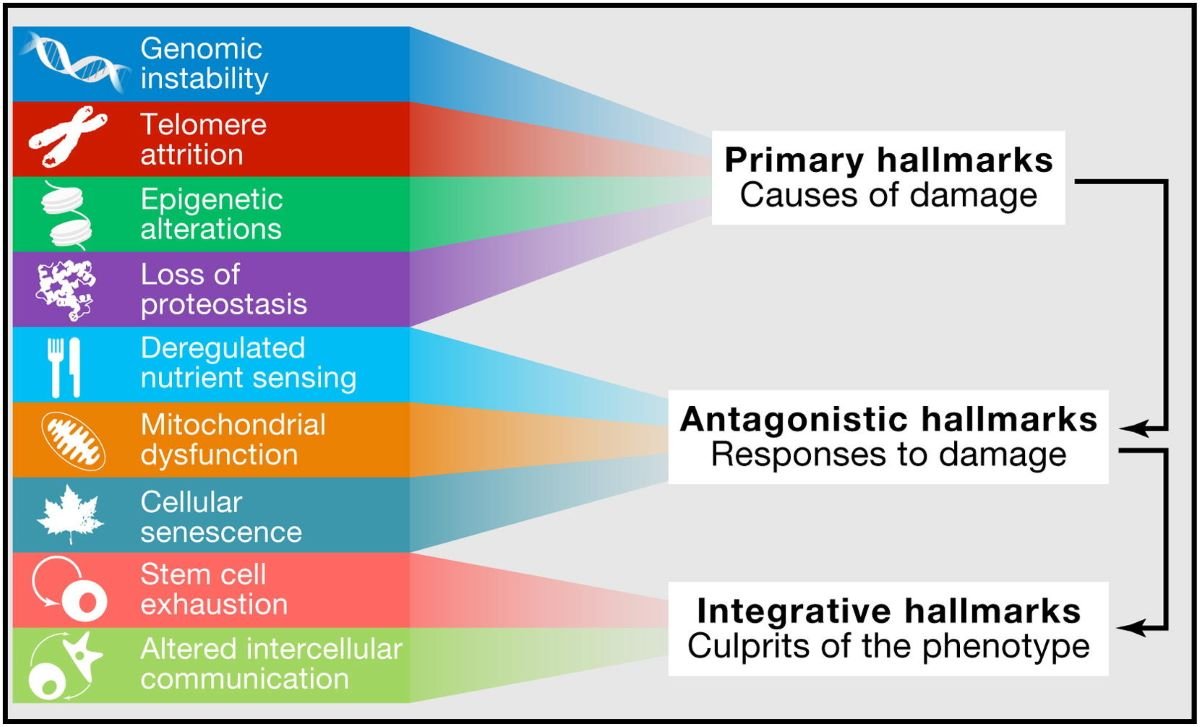
This article aims to explore the phenomenon of aging by examining the 12 hallmarks of aging that contribute to this process.
Understanding the underlying mechanisms of aging is crucial for developing strategies to mitigate its effects on human health and well-being.
By adopting an objective and evidence-based approach, this article will provide a comprehensive analysis of each hallmark’s impact on the aging process.
This investigation offers valuable insights into how these hallmarks interplay and influence overall aging, thereby catering to readers seeking a deeper comprehension of this complex biological phenomenon.
Why we age?
The underlying mechanisms and processes that contribute to the aging process are still not fully understood. However, extensive research has shed some light on the factors that play a role in why we age.
One of the main contributors is oxidative stress, which occurs when there is an imbalance between the production of reactive oxygen species (ROS) and the body’s ability to neutralize them with antioxidants. ROS can cause damage to cellular components such as DNA, proteins, and lipids, leading to functional decline and ultimately aging.
Another factor is telomere shortening. Telomeres are protective caps at the ends of chromosomes that shorten with each cell division. Eventually, when telomeres become critically short, cells enter a state of senescence or programmed cell death. This process leads to tissue dysfunction and contributes to the overall aging process.
Moreover, chronic inflammation has been implicated in aging. Inflammatory responses are initially beneficial for healing and defense against pathogens but can become harmful when they persist over time. Chronic inflammation damages tissues and disrupts normal cellular functions.
Additionally, mitochondrial dysfunction plays a significant role in aging. Mitochondria are responsible for producing energy within cells through oxidative phosphorylation. As mitochondria age or become damaged by various factors including oxidative stress, their function declines resulting in reduced energy production.
What are the 12 Hallmarks Of Aging?

One approach to understand the processes of aging is by examining the twelve identified characteristics that are associated with the progression of age-related decline. These hallmarks of aging provide a framework for comprehending the underlying mechanisms that contribute to aging at a cellular and molecular level.
- The first hallmark is genomic instability, which refers to an increased susceptibility to DNA damage and mutations as cells age. This can lead to impaired cellular function and increased risk of diseases like cancer.
- The second hallmark is telomere attrition, where the protective caps on the ends of chromosomes shorten over time, leading to cellular senescence and reduced regenerative capacity.
- Another hallmark is epigenetic alterations, which involve modifications in gene expression patterns without changing the underlying DNA sequence. These alterations can affect various biological processes and contribute to age-related phenotypes.
- Mitochondrial dysfunction, another characteristic, results from accumulated damage in mitochondria – cell organelles responsible for energy production – leading to decreased energy production and increased oxidative stress.
- Other hallmarks include loss of proteostasis (the ability to maintain protein homeostasis), deregulated nutrient sensing pathways, cellular senescence (permanent growth arrest), stem cell exhaustion, altered intercellular communication, immune system dysregulation, and chronic inflammation.
Understanding these hallmarks provides insight into the complex nature of aging and may pave the way for potential interventions aimed at mitigating age-related decline.
1. Altered intercellular communication
Altered intercellular communication is a characteristic of aging that affects various physiological processes, such as immune response and hormonal regulation.
As we age, our cells become less effective at communicating with each other, leading to disruptions in the normal functioning of the body. This decline in intercellular communication can have significant consequences on our health and well-being.
For example, a weakened immune system makes us more susceptible to infections and diseases. Hormonal imbalances can result in changes in mood, metabolism, and reproductive function.
Additionally, altered intercellular communication can also impact systemic blood factors, which play a crucial role in maintaining homeostasis within the body.
2. Cellular senescence

Cellular senescence, characterized by the cessation of cell division and the secretion of proinflammatory molecules, is a physiological state that arises from factors such as DNA damage, oxidative stress, and mutations. This process plays a significant role in aging and age-related diseases.
Senescent cells accumulate with age and their presence has been linked to tissue dysfunction and inflammation in various organs. The proinflammatory molecules secreted by these cells can disrupt tissue homeostasis and contribute to chronic inflammation, which is a hallmark of aging.
Additionally, senescent cells have been shown to negatively impact neighboring cells through altered intercellular communication. While cellular senescence serves as an important defense mechanism against cancer development, its accumulation over time can lead to detrimental effects on tissue function and overall health.
3. Chronic inflammation
Chronic inflammation is a key component of the aging process and is closely related to the accumulation of senescent cells and infectious pathogens. Termed ‘inflammaging,’ this phenomenon refers to the progressive increase in inflammation that occurs with age.
Inflammation is a complex biological response triggered by harmful stimuli, such as pathogens or tissue damage, but can become dysregulated in older individuals. The mechanisms underlying inflammaging involve a variety of factors, including alterations in immune cell function, increased production of pro-inflammatory molecules, and impaired resolution of inflammation.
Chronic inflammation contributes to the development and progression of many age-related diseases, such as cardiovascular disease, neurodegenerative disorders, and cancer.
4. Deregulated nutrient-sensing
Deregulated nutrient-sensing pathways play a crucial role in the development of age-related metabolic disorders and contribute to the decline in overall health with increasing age. These pathways, which are responsible for sensing and responding to nutrients, become less effective as an individual ages.
This decline in effectiveness can lead to dysregulation of metabolism and an increased risk of developing metabolic disorders such as type 2 diabetes.
One key aspect of nutrient-sensing pathways is their ability to regulate insulin signaling and glucose metabolism. With age, this regulation becomes impaired, leading to insulin resistance and elevated blood glucose levels.
Additionally, deregulated nutrient-sensing pathways can also disrupt lipid metabolism, leading to increased fat accumulation and dyslipidemia.
The deregulation of these pathways can have far-reaching consequences on an individual’s health. Not only do they contribute to the development of metabolic disorders, but they also affect other hallmarks of aging such as chronic inflammation and cellular senescence.
In conclusion, deregulated nutrient-sensing pathways are significant contributors to age-related metabolic disorders and the overall decline in health with advancing age.
5. Disabled macroautophagy
In the context of aging, the previous subtopic discussed deregulated nutrient-sensing as one of the hallmarks. This disruption in nutrient signaling pathways can contribute to cellular dysfunction and ultimately impact aging processes.
Building upon this, the current subtopic focuses on disabled macroautophagy, which is another hallmark of aging. Autophagy plays a crucial role in maintaining cellular homeostasis by removing damaged organelles and proteins.
However, with advancing age, macroautophagy becomes impaired, leading to an accumulation of dysfunctional components within cells. This impairment has been linked to several age-related diseases such as neurodegenerative disorders and cancer.
Trans-Reseratrol Powder, 5 Ounces
- Pure Trans Resveratrol Powder with Immune Natural Vitamin E (D-Alpha Tocopherol) from Micro Ingredients.
- 5 Ounce, (Resveratrol 500mg Per Serving), 2 in 1 Formula for Super Antioxidant, Micronized Powder for Better Absorption, Vegan Friendly.*
- Non-GMO, No Gluten, No Soy, No Tree Nuts, No Artificial Colors, No Flavors and No Irradiation.
⭐⭐⭐⭐⭐ | 4.6 (535 reviews)
6. Dysbiosis
Dysbiosis, characterized by an imbalance in the gut microbiome composition and function, has been associated with various pathological conditions and may have implications for overall health.
As individuals age, significant shifts occur in the gut microbiome, leading to a loss of ecological diversity and a decrease in beneficial microbes. These changes can contribute to dysbiosis, which is characterized by an altered ratio of beneficial to harmful bacteria in the gut.
Dysbiosis has been linked to several diseases including obesity, inflammatory bowel disease, and metabolic syndrome. The mechanisms underlying this association are not yet fully understood but are thought to involve alterations in microbial metabolites, immune responses, and intestinal permeability.
7. Epigenetic alterations

Epigenetic alterations have been identified as potential contributors to age-related changes in gene expression and overall epigenomic dysregulation. The dysregulation of the epigenome, specifically DNA methylation, tends to decrease with age. This global decrease in DNA methylation can lead to the activation of genes that should be turned off and vice versa.
Such epigenetic alterations have been implicated in various age-related diseases and conditions, including cancer, neurodegenerative disorders, and cardiovascular diseases.
Studies have shown that specific regions of the genome are particularly susceptible to age-related changes in DNA methylation patterns. Furthermore, it has been observed that certain environmental factors such as diet and lifestyle choices can influence these epigenetic alterations.
8. Genomic instability
Genomic instability is another hallmark of aging that contributes to the accumulation of mutations in our DNA. As mentioned earlier, errors during DNA replication and repair processes can lead to mutations. These mutations can disrupt the normal functioning of genes and have detrimental effects on cellular integrity and function.
Several factors contribute to genomic instability, including environmental exposures such as radiation and certain chemicals. Additionally, the gradual decline in cellular mechanisms responsible for maintaining genomic stability with age further exacerbates this problem.
Genomic instability has been linked to various age-related diseases, including cancer and neurodegenerative disorders. The accumulated mutations can alter critical signaling pathways, leading to uncontrolled cell growth or impaired neuronal function.
9. Loss of proteostasis
The loss of proteostasis is characterized by the accumulation of misfolded proteins and impaired protein degradation, leading to detrimental effects on cellular function and contributing to age-related diseases.
Proteins play crucial roles in various cellular processes, and maintaining their proper structure is essential for their functionality. However, with aging or in certain neurodegenerative disorders such as Alzheimer’s disease, proteins can become misfolded and form aggregates that are toxic to cells.
This impairment in proteostasis can result from a decline in the efficiency of protein folding mechanisms or an imbalance between protein synthesis and degradation pathways. The accumulation of these misfolded proteins disrupts normal cellular function, eventually leading to cell death and tissue damage.
Consequently, the loss of proteostasis is considered one of the hallmarks of aging and a prominent contributor to age-related diseases.
10. Mitochondrial dysfunction
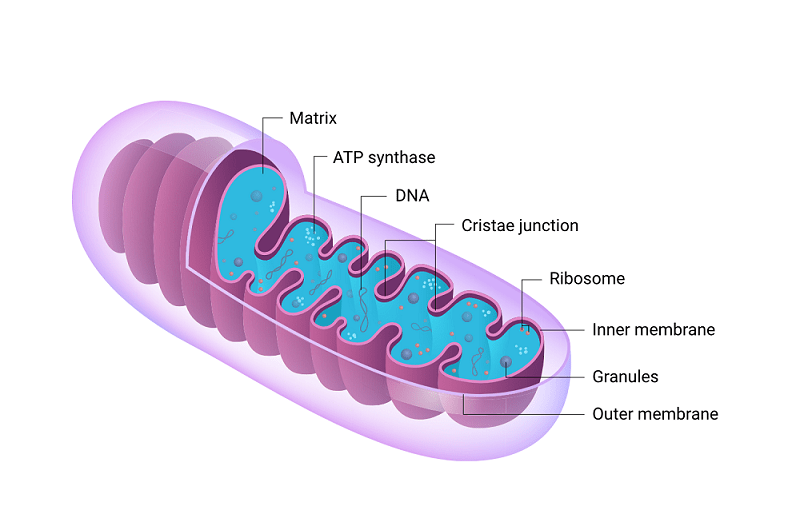
Another hallmark closely associated with aging is mitochondrial dysfunction. Mitochondria are essential for energy production and play a crucial role in various cellular processes. 1
However, over time, these organelles accumulate DNA mutations and oxidative damage, leading to impaired mitochondrial function.
This decline in mitochondrial efficiency can result in decreased ATP production and increased generation of free radicals. 2
Additionally, dysfunctional mitochondria can trigger cell death pathways and promote inflammation within the cell.
The consequences of mitochondrial dysfunction include reduced cellular energy levels, impaired tissue function, and heightened susceptibility to age-related diseases such as neurodegenerative disorders and cardiovascular diseases.
11. Stem cell exhaustion
Stem cell exhaustion, a phenomenon characterized by a decline in the number and regenerative capacity of stem cells with age, is closely associated with the accumulation of DNA mutations and oxidative damage within these cells. 3
As individuals age, their stem cells gradually lose their ability to divide and differentiate into specialized cell types. This decline in regenerative capacity can be attributed to several factors.
Firstly, the accumulation of DNA mutations over time leads to an increased risk of genetic errors during stem cell division, resulting in reduced functionality. Additionally, oxidative damage caused by reactive oxygen species impairs the function and survival of stem cells.
The combination of these factors contributes to the progressive depletion and dysfunctionality of stem cells, leading to impaired tissue regeneration and an overall decline in organ function with age.
12. Telomere attrition
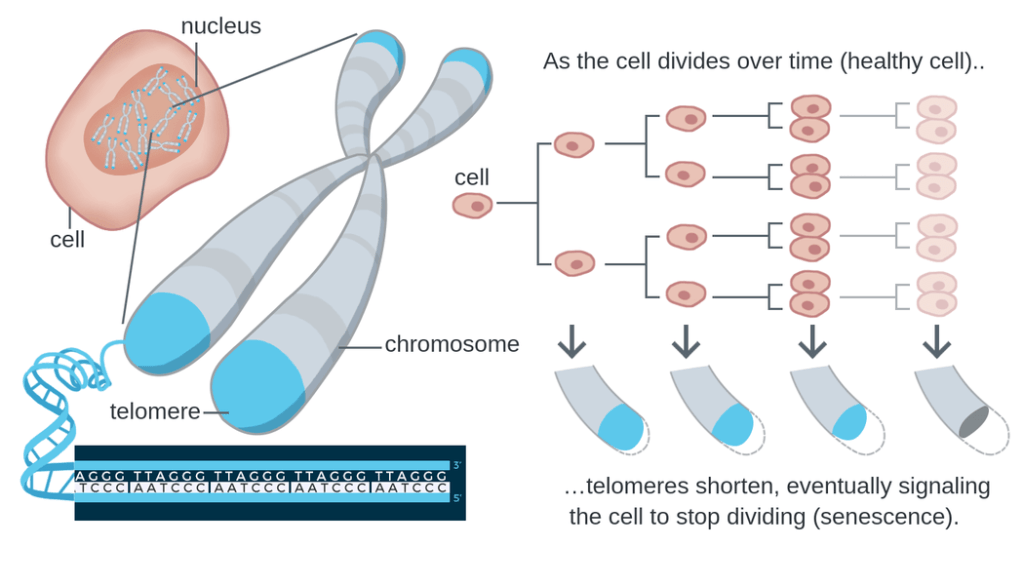
Telomere attrition, a key characteristic of cellular senescence, is driven by the gradual shortening of protective caps on chromosome ends with each cell division.
Telomeres play a crucial role in maintaining genomic stability and preventing chromosomal fusion or degradation. However, due to the inability of DNA polymerase to fully replicate the ends of linear chromosomes, telomeres progressively shorten over time.
This process is accelerated by various factors such as oxidative stress and inflammation.
As telomeres reach a critically short length, they activate cell cycle arrest pathways, leading to cellular senescence or apoptosis. Telomere attrition has been implicated in aging and age-related diseases, as it limits the replicative capacity of cells and affects tissue homeostasis.
Related: Top 10 Best Supplements for Telomere Protection & Elongation
Frequently Asked Questions
Bottom Line
In the realm of aging research, understanding the underlying mechanisms that drive the process of aging is crucial. Scientists have identified 12 hallmarks of aging, which are a set of cellular and molecular processes that contribute to age-related decline.
These hallmarks include genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, altered intercellular communication, immunosenescence, chronic inflammation, and metabolic dysfunction.
By studying these hallmarks and their interactions with each other, researchers hope to develop interventions that can slow down or even reverse the effects of aging.
In conclusion, the 12 hallmarks of aging provide a comprehensive framework for understanding the complex process of getting older. Each hallmark represents a specific aspect of cellular and molecular functioning that undergoes changes as we age.
References:
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3513836/ ↩︎
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4145906/ ↩︎
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4214082/ ↩︎
Read Next




